lithium battery
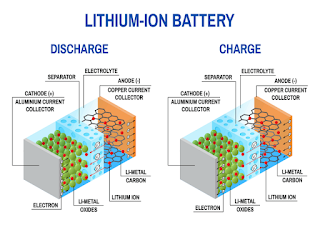
Today there are different kind of batteries based on the application for which they will be used. Actually. Li-ion batteries are the most popular. Let's have a closer look to the internal structure of a lithium ion cell in order to understand how it works:
If we open a battery pack and break it down we can see different layers of chemical compounds inside it. Lithium ion batteries work on a concept associated with metals called electrochemical potential. Electrochemical potential is the tendency of a metal to lose electrons. In fact, the very first cell developed by Alessandro Volta more than 200 years ago was based on the concept of electrochemical potential. Lithium is the metal which has the highest tendency to lose electrons and it is used in lithium ion cells. It has only one electron in its outer shell and it wants always to lose this electron. Due to this reason, pure lithium is a highly reactive metal and it even reacts with water and air. The trick of a lithium battery operation is the fact the lithium, in its pure form, is a reactive metal but when it is a part of a metal oxide is quite stable. If we assume that somehow we are able to separate the lithium atom from this oxide, since its instability, it will form a lithium ion and an electron. If we can provide two different paths for the electron and lithium ion flow between the lithium and the metal oxide, the lithium atom will automatically reach the lithium oxide part. During this path we have produced electricity from the electron flow through the one path. Hence, essentially, we can produce electricity from this metal oxide if we firstly separate the lithium atom and then we guide the electron separate from the atom through an external circuit.