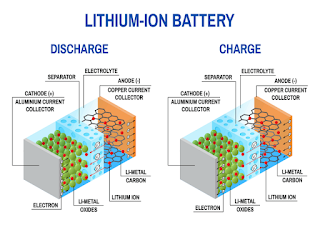
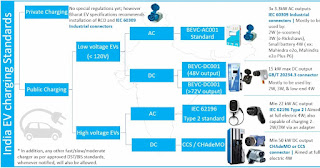
Energy Materials Research Centre (EMRC)

RESEARCH INTERNSHIP - Renewable Energy, Energy Storage, Environmental Protection, Advanced Agriculture DIPLOMA COURSE - Diploma In Renewable Energy Application, CERTIFICATE COURSE, Commercialization Of Sodium-Ion Batteries ADDON COURSE - Lithium-Ion Battery Pack Production Line Professional Course We're focused on research excellence, effective industrial partnerships and creative engineering education. Best Teacher Good teacher include skills in communication, listening, collaboration, adaptability, empathy and patience. Library & Book Store The library’s collection of books, journals, and other materials be so constituted and organized to provide direct support to all Internship and Research Students will work on a research project mentored by an EMRC researcher, gain valuable skills for success in their chosen field.